- Moody’s Sees Drug Price Increases Slowing (healthleadersmedia.com)
Despite the slowing rate, rising drug costs and potential changes to Medicare 340B payments for outpatient drugs would further reduce hospitals' margins...Inpatient drug costs will continue to rise for not-for-profit hospitals, but the pace will slow under growing scrutiny of drug makers' pricing practices, Moody's Investors Service said...Drug costs have outpaced hospital revenue growth in recent years...the median growth rate for supply costs, which include drugs, slowed between 2015 and 2016. But the gap between how fast supply costs grew versus (revenue growth) revenues grew widened...Price increases in recent years were extraordinarily high for certain branded hospital inpatient drugs, but drug manufacturers are pulling back on these increases...On the generic drug side, we expect that some of the pressure will ease as the U.S. Food and Drug Administration approves more generic drugs for the first time...The proposed reduction of Medicare Part B outpatient drug reimbursement to 340B hospitals by roughly 30% would represent another headwind for hospitals already facing pressure...
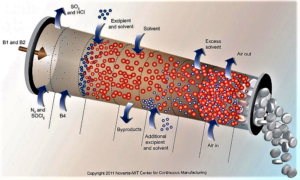
- FDA calls for industry input on continuous manufacturing guidelines (biopharmadive.com)
The Food and Drug Administration plans to develop clearer guidelines around the adoption of continuous manufacturing and has asked industry stakeholders for input on how best to design a regulatory framework...The regulator has advocated companies make the switch from traditional batch production to continuous manufacturing, which to a large extent hasn't been widely adopted by drugmakers despite the potential benefits to speed and reliability...continuous manufacturing can reduce the risk of manufacturing failures and potentially help prevent drug shortages from developing — areas that have been in focus as the agency steps up its oversight of pharmaceutical production facilities...Pharma has been loath to change over from batch production, which has served the industry for decades...Continuous manufacturing techniques — already adopted in many other industries — promise to shorten production times significantly, while reducing the amount of human intervention needed throughout...The FDA hopes providing a "framework of principles" will help drugmakers navigate CM adoption and implementation...
- FDA steps up oversight of cell therapies (biopharmadive.com)
The Food and Drug Administration is stepping up its enforcement of the existing rules covering stem cell therapies in order to protect patients from unproven or potentially dangerous treatments...Under "a comprehensive policy framework" that will be put into place next quarter, individual providers and companies will be able to gain approval for products and procedures through a lower-cost and more streamlined process...A new working group will pursue unscrupulous clinics through legally enforceable means in order to protect public health...Patients desperately seeking solutions for potentially fatal diseases can get to a point that they will clutch at any solution offered...FDA Commissioner Scott Gottlieb's statement shows an awareness of the potential for "unscrupulous actors" in the field, and their impact both on vulnerable people and on the reputation of sound researchers and companies..."These dishonest actors exploit the sincere reports of the significant clinical potential of properly developed products as a way of deceiving patients and preying on the optimism of patients facing bad illnesses. This puts the entire field at risk. Products that are reliably and carefully developed will be harder to advance if bad actors are able to make hollow claims and market unsafe science. To make sure the agency is separating the promise from the unscrupulous hype, we are stepping up our enforcement activity in this area," said Gottlieb...
- FDA Details Plans for More Efficient Inspections, Facility Evaluations
The US Food and Drug Administration's Center for Drug Evaluation and Research and Office of Regulatory Affairs will soon launch an effort to streamline the two offices' inspection and facility evaluation efforts...CDER Director Janet Woodcock and Associate Commissioner for Regulatory Affairs Melinda Plaisier said it is vital that the two offices quickly implement the plan in order to meet commitments under the recently reauthorized user fee agreements, specifically citing the agency's promise to communicate final inspection classifications to generic drugmakers within 90 days of an inspection beginning in October 2018...We plan to operationalize the plan in the fall of 2017 for nearly all human drugs...FDA details the plan—which includes specific operating models for pre- and post-approval inspections, surveillance inspections and for-cause inspections—in a 20-page white paper obtained by Focus entitled Integration of FDA Facility Evaluation and Inspection Program for Human Drugs: A Concept of Operations...
- FDA warns ‘critical’ drug shortages possible after Hurricane Maria battered Puerto Rico (usatoday.com)
Patients could experience "critical shortages" of key pharmaceuticals, the U.S. Food and Drug Administration is warning after Hurricane Maria brought Puerto Rico's drug manufacturing industry to a standstill...The FDA said...it is taking active measures to help redirect production and preserve existing treatments to avoid a ballooning health crisis from Maria's destruction...The agency did not identify any specific medications that could be at risk of a shortfall, and a spokesperson was not immediately available to provide details...But there are "several" cases where "we may soon face critical shortages if we don’t find a path for removal or ways to get production back up and running," FDA Commissioner Scott Gottlieb said in a statement...Some companies are beginning to move product off of the island, and they’ve been communicating with the FDA about that and what potential challenges and limiting factors they see ahead...Drugs made on the island include AstraZeneca's cholesterol treatment Crestor, Abbvie arthritis drug Humira and Johnson & Johnson-owned HIV drug Prezista. Those three companies have said supplies of their drugs are in good shape...the catastrophic storm wiped out electricity for the entire island, devastated telecommunications and made travel nearly impossible for many employees of the island's nearly 50 pharmaceutical factories...
- F.D.A. Accuses EpiPen Maker of Failing to Investigate Malfunctions (nytimes.com)
The Food and Drug Administration...accused the drugmaker Pfizer of failing to properly investigate reports of malfunctioning EpiPens, including incidents when patients died or became severely ill after the device failed to work. Pfizer manufactures the EpiPen, which treats allergic reactions, for the drugmaker Mylan...In a warning letter...the agency said Meridian Medical Technologies, which is a unit of Pfizer, did not adequately look into problems with a crucial component of the EpiPen — the mechanism on the device that insures that it fires and delivers the proper dose of epinephrine, which stops an allergic reaction... the company failed to conduct a proper investigation even though it received numerous complaints about problems with activating the device. "Our own data show that you received hundreds of complaints that your EpiPen products failed to operate during life-threatening emergencies, including some situations in which patients subsequently died...
- Feds seize smallpox vaccine from clinic injecting it into cancer patients (theverge.com)
The US Food and Drug Administration has stopped a California company from continuing to inject the smallpox vaccine into the tumors of cancer patients...US marshals seized five vials of the smallpox vaccine from San Diego-based StemImmune Inc, which was using them as part of an unproven method for treating tumors...he vaccine is not commercially available and is only reserved for people who are at very high risk for developing smallpox, so it’s not clear how StemImmune got the vials to begin with. Luckily, the vaccine is not made from the actual smallpox virus and cannot give anyone the disease...It was...not meant to be used the way StemImmune was doing so, which was by mixing it with stem cells that come from body fat and injecting it into the tumors of patients at California Stem Cell Treatment Centers in Beverly Hills and Rancho Mirage...The FDA is encouraging consumers who have tried the treatment and had bad effects to use its MedWatch Adverse Event Reporting program.
- Many drug companies fail to conduct timely safety checks on medicines after FDA approval (reuters.com)
In the rush to approve new medicines, the U.S. Food and Drug Administration often requires drug companies to study possible side effects and alternative doses for medicines once they hit the broader market...A new analysis in the New England Journal of Medicine concludes that, in many cases, that’s not being done...among the 614 studies mandated in 2009 and 2010, 20 percent were never started and 9 percent have been delayed...When drugs are approved, the trials are usually small and short-term, and some side effects may not emerge until the post-marketing phase...The problem is, the faster you get them on the market, the more open questions there are about their safety or the best dose...In some cases, the FDA has simply dropped a requirement for a postapproval study without giving a reason...
- Why the Biosimilar Drug Revolution Hasn’t Arrived (bloomberg.com)
In a word: patents...The Biologics Price Competition and Innovation Act (2010) was...part of the Affordable Care Act. Its essential goal was to infuse competition and lower the prices of drugs that were made from living cells -- so-called biologics...Until the 2010 law, biologics had no fear of competition -- there was no legal way to introduce generic versions into the market -- so they were able to maintain their monopoly price even after their patents expired. The BPCIA was intended to establish mechanisms within the Food and Drug Administration, the Patent and Trademark Office, and the courts that would allow the introduction of "biosimilars." These drugs weren't exact replicas of biologics, but were similar enough, and safe enough, to be used instead of the brand-name drugs...Here we are seven years later. Guess how many biosimilars have made it to market?..Two...companies are forced to fight through thickets of patents to get a biosimilar to market, a law that was supposed to save the U.S. billions will continue to do just the opposite: make it easy for biologic makers to maintain unwarranted monopolies...
- Libertarian billionaire Peter Thiel funds “unethical” offshore human test of herpes vaccine, skirting FDA rules (salon.com)St. Kitts Launches Probe Of Herpes Vaccine Tests On U.S. Patients (khn.org)
Defying U.S. safety protections for human trials, an American university and a group of wealthy libertarians...are backing the offshore testing of an experimental herpes vaccine...Peter Thiel, invested $7 million in the ongoing vaccine research...Southern Illinois University also trumpeted the research and the study’s lead researcher...Neither the Food and Drug Administration nor a safety panel known as an institutional review board..monitored the testing of a vaccine its creators say prevents herpes outbreaks. Most of the 20 participants were Americans with herpes who were flown to the island several times to be vaccinated, according to Rational Vaccines...“What they’re doing is patently unethical,” said Jonathan Zenilman, chief of Johns Hopkins Bayview Medical Center’s Infectious Diseases Division. “There’s a reason why researchers rely on these protections. People can die.”...The risks are real. Experimental trials with live viruses could lead to infection if not handled properly or produce side effects in those already infected...