- FDA Guidance on Biosimilar Substitution Needs Work, Commenters Say (bna.com)
The FDA needs to clarify its standards for showing a biosimilar drug is interchangeable with the original biologic and how the biosimilar should be named and labeled, commenters said in response to an agency draft guidance...The Food and Drug Administration draft...described how an applicant can establish that a biosimilar, a highly similar, less expensive version of an FDA-approved biologic drug, can be substituted for the biologic on which it is based without a physician’s approval…If you’re a biosimilar applicant, you’re happy the FDA has provided the guidance, but you think it’s asking for too much information...If you’re the owner of the patent for the original biologic, you strongly support the need for the switching studies the FDA requires in order to show that switching from the original biologic to the biosimilar is safe. If you’re an organization that represents patients, you want assurances the interchangeable biosimilar is safe and effective for each condition for which the original biologic is approved...
- Failure to Reauthorize User Fee Programs Would Result in About 3,000 FDA Layoffs (raps.org)
Representatives from the biotechnology, medical device and generic drug industries told members of the Senate Committee on Health, Education, Labor & Pensions...that if the five-year user fee programs are not reauthorized, the US Food and Drug Administration would likely see more than 3,000 job cuts...Those layoffs...would significantly curtail new medical product approvals for pharmaceutical, biotech and medical device companies and likely delay research...Senators on both sides of the aisle also criticized Trump’s budget proposal on Tuesday, which would cut billions from the National Institutes of Health and replace congressional appropriations at FDA with more user fees...Representatives from industry groups BIO, AdvaMed and AAM reiterated to the committee what FDA officials told the House Energy & Commerce Committee late last month: that a failure to act on the already-agreed-to reauthorizations would be devastating...
- Gilead snaps up Sarepta’s priority review voucher for $125M (biopharmadive.com)
Hoping to speed up future regulatory review for its drug candidates, Gilead has agreed to buy a priority review voucher from Sarepta Therapeutics for $125 million...Sarepta had obtained the voucher — a transferrable credit designed to speed review by the Food and Drug Administration by four months — for winning approval of its disease-modifying treatment for Duchenne muscular dystrophy last year...Sale of the voucher, albeit for a lower price than some had expected, will bolster Sarepta's cash position and help fund clinical development of Sarepta's pipeline and manufacturing scale-up…For Gilead, the voucher could be used to speed review for one of its several late-stage candidates. The $125 million is a relative bargain as well, compared to both the recent prices paid by Sanofi and AbbVie as well as to Gilead's large cash hoard.
- FDA calls for switching studies in draft interchangeability guidelines (biopharma-reporter.com)
The FDA expects biosimilar developers to provide data from switching studies to demonstrate interchangeability with a reference biologic in draft guidance...The recommendation – which is set out in long awaited draft guidance today – is that sponsors should submit data from a switching study, or studies, to the Food and Drug Administration in order to deem a biosimilar interchangeable with its reference product…The main purpose of a switching study or studies is to demonstrate that the risk in terms of safety or diminished efficacy of alternating or switching between use of the proposed interchangeable product and the reference product is not greater than the risk of using the reference product without such alternation or switch…prior to today’s draft guidance the US agency had not defined how a sponsor must go about generating such proof (interchangeability)...
- FDA sends warnings letters to companies making cancer cure claims (drugstorenews.com)
The Food and Drug Administration...posted warning letters addressed to 14 U.S.-based companies allegedly illegally selling more than 65 products that fraudulently claim to prevent, diagnose, treat or cure cancer. The products are marketed and sold without FDA approval, most commonly on websites and social media platforms...Consumers should not use these or similar unproven products because they may be unsafe and could prevent a person from seeking an appropriate and potentially life-saving cancer diagnosis or treatment…The illegally sold products cited in the warning letters...include a variety of product types, such as pills, topical creams, ointments, oils, drops, syrups, teas and diagnostics (such as thermography devices). They include products marketed for use by humans or pets that make illegal, unproven claims regarding preventing, reversing or curing cancer; killing/inhibiting cancer cells or tumors; or other similar anti-cancer claims.
- Trump FDA Nominee Wants Lower Drug Costs With More Generics (bloomberg.com)Trump selects Scott Gottlieb as FDA nominee (cnn.com)
President Donald Trump’s pick to head the U.S. Food and Drug Administration is among the most vigorous advocates of lowering drug costs by approving cheap generics faster, an initiative aimed directly at the profit centers of major companies...Scott Gottlieb, a former FDA deputy commissioner, would make streamlining approvals his top priority...He’s particularly focused on complex medications that combine old drugs with newer delivery devices, as well as those with unusually complicated formulations...The administration could make the changes without Congress passing a law...But it also risks angering companies that have considerable legal firepower to defend their money-makers...Getting more generics to market faster could save Americans billions of dollars a year...Absent a new law, the FDA could do much to speed approval of generics. It could devise broad guidelines for copies. And the agency could become swifter in approving drug-device combos, finding ways to allow slight differences so long as they wouldn’t confuse patients...
- President’s Pledge
- Complicated Cures
- Pricey Pens
- EpiPen Dispute
- Trump is promising big changes at the FDA — here’s how drugs are approved today (businessinsider.com)
At least one of President Trump's possible picks to head the Food and Drug Administration has a radical idea for when drugs should come to market...Jim O'Neill, managing director at Mithril Capital, has said that he is in favor of approving drugs that are proven to be safe, even before they're shown to be actually effective...Regardless of who Trump picks in the end, his interest in cutting regulation at the FDA is clear..."We're going to be cutting regulations at a level that nobody's ever seen before," Trump said in a meeting with pharma executives...As it exists right now, the FDA approval process can be a long and expensive…
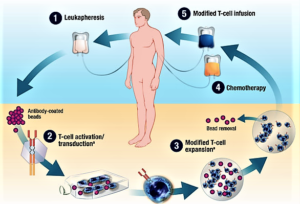
- The First CAR-T Drugs Have Left the Gate (fool.com)
Investors should keep an eye on this promising way to treat cancer...For all the talk about biotechs being nimble, it's a big pharma that looks like it'll be the first company to launch a chimeric antigen receptor T-cell (CAR-T) product...Novartis announced last week that the Food and Drug Administration accepted its application to market tisagenlecleucel-T...in patients with B-cell acute lymphoblastic leukemia who are relapsed and refractory to other therapies...A few days later, Kite Pharma completed its application for axicabtagene ciloleucel...Kite's application could be accepted early, putting it less than two months behind Novartis…Since CAR-T therapies are personalized treatments that have to be made individually for each patient, they're likely to be expensive to produce and therefore require a premium price. The first company to get a CAR-T therapy approved will set the price, which later companies may have to match unless they can justify a higher price with higher efficacy...With prices that will probably exceed those of current cancer treatments, investors should expect some pushback from insurers. One way Novarits and Kite can get around the cost issue is by offering money-back guarantees...Kite's and Novartis' CAR-T therapies are just the tip of the iceberg for this new way to treat cancer...
- Trump’s first budget seeks to slash $6B from NIH, raise FDA user fees (fiercebiotech.com)
President Donald Trump’s first budget will take $5.8 billion away from the National Institutes of Health, around 20% of its total, with FDA user fees also set to rise as biopharmas should "pay for their share."...The budget cut to the NIH...had around $30 billion in funds last year...The NIH...got a funding boost just last year when the 21st Century Cures Act was passed, a law that allowed the Institute an extra $4.8 billion in funding over the next decade...There was no direct mention of FDA cuts, but use fees are set to potentially double..Recalibrates Food and Drug Administration medical product user fees to over $2 billion in 2018, approximately $1 billion over the 2017 annualized CR level, and replaces the need for new budget authority to cover pre-market review costs. To complement the increase in medical product user fees, the Budget includes a package of administrative actions designed to achieve regulatory efficiency and speed the development of safe and effective medical products...
- FTC accuses Shire subsidiary of delaying generic rivals (biopharmadive.com)
The Federal Trade Commission...accused a Shire plc subsidiary of filing dozens of "sham" petitions with the Food and Drug Administration in an effort to delay generic competition to its branded prescription drug Vancocin HCI...the FTC alleged that Shire ViroPharma filed 43 citizen petitions with the FDA (along with 3 lawsuits) over a seven-year period, knowing that the FDA typically waited to approve generic drugs until it had resolved any outstanding citizen petitions...a deliberate attempt to maintain a monopoly...ViroPharma’s efforts led consumers and other purchasers to pay hundreds of millions of dollars more for the drug.