- China updates national drug list, adding some blockbuster Western meds (fiercepharma.com)
China has updated its list of medicines covered by national medical insurance, adding some new drugs with a focus on pediatrics and major illnesses such as cancer, hepatitis, and renal and cardiovascular diseases…The overhaul, the first since late 2009, saw the number of "Western-style" and traditional Chinese medicines included in the list grow by 15% to 2,535, among which 1,297 are Western-style meds, an 11% increase...The additions include some blockbuster meds like tenofovir, an antiviral drug to treat hepatitis B and HIV...and cancer drug gefitinib...China’s own non-small cell lung cancer med icotinib…The ministry (Ministry of Human Resources and Social Security) also put 45 drugs on a “to-be-negotiated” list, half of which are targeted cancer therapies...inclusions of new drugs would reduce the financial burden on patients and help support innovations in China's pharmaceutical market...The National Reimbursement Drug List names all the drugs covered by the insurance program, some in full and others partially. Patients must pay the full price out of pocket for those drugs outside the list, which means a huge financial burden, especially when new but more effective drugs are not covered..
- NASP Statement on Recent Media Reports About “Specialty Pharmacies” (madmimi.com)
Mischaracterization of Specialty Pharmacies: NASP (National Association of Specialty Pharmacy) Corrects the Record….published recently in the New York Times mischaracterize specialty pharmacies. The fact that a company calls itself a specialty pharmacy does not make it so. Simply stated, charging high prices for medications does not define a specialty pharmacy…A specialty pharmacy is a state-licensed pharmacy that solely or largely provides only medications for people with serious health conditions such as cancer, hepatitis, rheumatoid arthritis, HIV/AIDs, multiple sclerosis, organ transplantation, or bleeding disorders…specialty pharmacies are often accredited by independent third parties such as URAC (Utilization Review Accreditation Commission) or the Accreditation Commission for Health Care…Specialty pharmacies provide medications and related high touch health care services to seriously ill people who need complex medications, assistance in using these medications properly and not infrequently, assistance with reimbursement. Specialty medications have a complex profile, often requiring intensive patient management as well as special handling and administration assistance…
- Engineers 3D Print Tissue That Mimics How The Human Liver Functions (forbes.com)
Engineers at the University of California...say they have successfully 3D printed life-like liver tissue that simulates how the human liver functions and is structured. The researchers say the tissue could be used as a platform for drug screening...In the case of Federal Drug Administration approval for a drug, on average it takes around 11 to 14 years and $2.6 billion to get a drug to market...Around 90% of drugs don’t pass animal tests or human clinical trials. In the case of the new 3D printed tissue, the researchers say pharmaceutical companies could use the tissue as platform in the lab to focus on drugs that appear to be more promising and eliminate drugs that have less efficacy...To create the liver tissue that mimics real human liver tissue, the engineers created a diverse combination of liver cells and supporting cells systematically organized in a hexagonal pattern under a microscope. But to print that complex tissue, they needed a 3D printer that could accommodate the 3D micro-structures found in biological tissue. The team created their own bioprinting tech in the lab capable of reproducing the elements and features of the tissue…I think that this will serve as a great drug screening tool for pharmaceutical companies and that our 3D bioprinting technology opens the door for patient-specific organ printing in the future. The liver tissue constructed by this novel 3D printing technology will also be extremely useful in reproducing in vitro disease models such as hepatitis, cirrhosis and cancer...
- Price fixing: PBMs push for lower prices of PCSK9 inhibitors (mmm-online.com)
Gilead Sciences' decision to slap Sovaldi,…with an $84,000 price tag was widely considered a turning point for payers and pharmacy benefit managers…the months leading up to the approvals this summer…negotiations were already under way between those drugmakers and Express Scripts,…PBMs are becoming increasingly savvy and hard-nosed about which drugs they will cover and what they are willing to pay for them… PBMs want to position [themselves] so they are advocating on behalf of their customers. Their customers are worried...
- Pharma tracks consumer lawsuit in Arizona for clues to future liability (statnews.com)
In a split decision for the pharmaceutical industry, the Arizona Supreme Court issued an opinion that has drug makers both encouraged and worried as they track the progress of a case that may have an outsized impact on consumer lawsuits filed in the state...The case was brought by Amanda Watts, a young woman who claimed she developed lupus and hepatitis after taking an acne treatment made by Medicis Pharmaceutical...She contended the company failed to provide proper side effect warnings. A state appeals court sided with Watts, which prompted Medicis to try to have the decision overturned...Watts has argued over two points that alarm drug makers...The loss of uniformity in liability standards for prescription medicines will subject pharmaceutical manufacturers to fundamentally different standards of liability in each state...(1) A key argument Watts made is that a long-standing industry defense against consumer lawsuits conflicted with another state law about deciding who may be at fault when there is harm. Known as the learned intermediary, this defense says drug makers cannot be held liable if a consumer suffers harm from a medicine — so long as all risk information was appropriately conveyed to the patient’s physician...The state Supreme Court gave drug makers a lift by deciding that the learned intermediary is a legitimate defense...(2) Meanwhile, the pharmaceutical industry will be watching whether Watts wins her argument over consumer fraud violations. Although drug makers argue their customers are actually physicians, the state Supreme Court opined that a direct transaction between a drug maker and a patient is not required for a consumer to claim fraud in the event that misrepresentation resulted in injury...It could be a game changer...
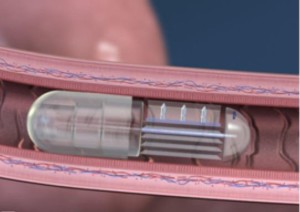
- Robotic Pills Could Simplify Administration of Biologic Drugs (pharmacytimes.com)
robotic pill may be the first oral agent to successfully traverse the acidic digestive environment to deliver biologic drugs that, to date, could only be delivered via injection. The pill would facilitate..administration of complex biotech drugs.. ingestible technology increasing convenience...supporting adherence...communicates to caretakers and providers whether a patient has taken his or her doses..