- Drug Supply Chain Security: OIG Finds Pharmacies Received Most Tracing Information (raps.org)
A new report from the Department of Health and Human Services’ Office of Inspector General found that among a sample of 40 pharmacies, all received at least some of the required drug product tracing information from their supply chain partners, though many said they are not reviewing the information...Under the Drug Supply Chain Security Act, dispensers, including independent, chain and hospital pharmacies, are required to receive complete tracing information before accepting ownership of a drug shipped from a manufacturer or wholesaler...“Although dispensers are generally implementing the requirements for drug product tracing, missing information and a lack of awareness of DSCSA requirements raise concerns that a complete tracing record for a drug product may not always be available to support investigations of suspect and illegitimate drug products in the supply chain,” OIG said...OIG recommends (and FDA concurred) that FDA offer educational outreach to dispensers of drugs to ensure they understand their responsibilities in receiving complete tracing information from supply chain partners before accepting ownership of such pharmaceuticals...
- HHS watchdog office steps up focus on drug pricing (statnews.com)
Seeking to address rising concerns about prescription medicines, the watchdog arm of the Department of Health Human Services is adding to its list of pharmaceutical issues to be examined...The...Office of Inspector General...is trying to widen its focus as questions accelerate about drug costs and the sources for some medicines, according to a new work plan released today...the new issues that are being studied: the amount of medicine that may be wasted when cancer treatments...are distributed in single vials; the amount of money the government might save in the Medicare Part B program if rebates were tied to inflation; and questionable billing for topical medicines that are compounded and covered by Medicare Part D...The OIG is devoting more resources and “drilling down” as concerns rise over drug pricing and accompanying billing patterns...Prescription drug oversight is a top priority…
- New OIG Report Shows Hospitals’ Huge 340B Profits from Medicare-Paid Cancer Drugs (drugchannels.net)Part B Payments for 340B-Purchased Drugs (oig.hhs.gov)
..Office of Inspector General (OIG) has just released another eye-opening report on the 340B drug discount program: Part B Payments For 340B-Purchased Drugs.…The report documents how 340B-eligible hospital outpatient departments earn tremendous profits from the Medicare Part B program…OIG focuses on how the Medicare program could save money by sharing in these mega-profits. As usual, we don’t know if needy patients are benefiting from hospitals’ excess Medicare 340B profits. The OIG even reminds us three times in its report: “The 340B statute does not restrict how covered entities may use these funds.” Unfortunately, the Health Resources and Services Administration’s recent Omnibus Guidance didn’t bother to require that hospitals use 340B funds help the neediest patients access valuable medicines. Money, so they say, is the root of all evil today.
- HHS won’t stop using Yammer despite security warning at VA (medcitynews.com)
Department of Health and Human Services has no plans to curtail use of enterprise social network Yammer, despite a scathing report detailing serious security risks associated with that platform at the Department of Veterans Affairs…VA’s Office of Inspector General issued a report calling Microsoft-owned Yammer "unapproved," … we found that it had vulnerable security features, recurring website malfunctions, and users engaged in a misuse of time and resources,"..
- Understanding CMS’s Surprising Reimbursement Cut for 340B Hospitals (drugchannels.net)
Centers for Medicare & Medicaid Services shocked everyone with a proposal altering a small part of the 340B Drug Pricing Program. CMS proposed reducing reimbursement for certain Medicare Part B drugs purchased by 340B-eligible hospitals: from Average Sales Price plus 6% to ASP minus 22.5%. Hospitals will also have to identify 340B claims with a new modifier...CMS explains its rationale for reducing reimbursement to hospitals. It references key studies from the Office of Inspector General, the Government Accountability Office, and the Medicare Payment Advisory Commission...The new ASP-22.5% reimbursement figure is based on a MedPAC study. It estimated that hospitals in the 340B program receive a minimum discount of 22.5% of the Average Sales price (ASP) for drugs paid under the outpatient prospective payment system…Three important items to note:
- The proposal would reduce patients’ coinsurance obligations.
- Manufacturers would not gain from this proposal. CMS designed the proposal to be budget neutral.
- The proposal does not address contract pharmacies.
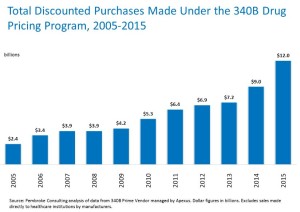
- 340B Purchases Hit $12 Billion in 2015—and Almost Half of the Hospital Market (drugchannels.net)
According to new data...discounted purchases made under the 340B Drug Pricing Program hit $12 billion in 2015. That’s a whopping 67% higher than the 2013 figure. I estimate that the undiscounted value of these purchases exceeds $17 billion...Most 340B purchases are made by hospitals. My...number-crunching below reveals that hospitals now receive 340B discounts on more than 44% of their drug purchases...two years ago, the 340B program is taking over the hospital market...How much of this money goes to uninsured and needy patients? No one knows, and the hospitals aren’t saying much...340B purchases have been growing much, much more quickly than have hospitals’ total drug purchases. From 2005 to 2015, total hospital drug purchases grew by 31%, compared with the 400%+ growth in the total 340B purchases...340B has infiltrated almost half of the hospital market...The 340B program is highly controversial, partly because the 340B legislation does not specify or restrict how covered entities should utilize funds generated by the program. Hence, it’s troubling to see that uncompensated care as a percentage of hospitals’ total expenses has remained at about 6% for many years, despite booming 340B purchases...Defenders of the 340B status quo argue that covered entities should be able to use discounts to reduce their cost of operations, without any transparency or accountability...The Office of Inspector General has documented how 340B-eligible hospital outpatient departments earn tremendous profits from the Medicare Part B program. Gross profit margins are about 60% compared with 3% to 4% for a non-340B outpatient program...
- 5 ways OIG checks hospital safety (healthcareitnews.com)
New this year is quality reporting data…The Office of Inspector General in the Department of Health and Human Services is charged with overseeing the agency's programs to make sure they function efficiently and safely…OIG recently released its work plan for 2016…Here, among the vast responsibilities assigned to the office are five items OIG will check to ensure hospitals provide quality care and maintain safety:
- CMS validation of hospital-submitted quality reporting data
- Hospitals' contingency plans for protecting data in the EHR
- Hospital preparedness and response to high-risk infectious diseases
- Long-term-care hospitals – adverse events in post-acute care for Medicare beneficiaries
- Inpatient rehabilitation facilities – adverse events for Medicare beneficiaries
- The Booming 340B Contract Pharmacy Profits of Walgreens, CVS, Rite Aid, and Walmart (drugchannels.net)
Pharmacies continue to ride the 340B Drug Pricing Program’s explosive growth...Our latest...analysis finds that nearly 20,000 pharmacy locations now act as a contract pharmacy for the hospitals and other healthcare providers that participate in the 340B Program. Fewer than 3,000 pharmacy locations were in the program in 2010...Large retail pharmacy chains' rapid expansion into 340B suggests superior profits...How many prescriptions do contract pharmacies provide at discounted prices to uninsured, underinsured, and low-income patients? Are pharmacies engaged in profit-sharing agreements with 340B hospitals? Are they earning fees that far exceed fair market value standards? Who is really benefiting from the contract pharmacy boom?...For instance, an Office of Inspector General study of 340B contract pharmacies found that two out of three hospitals did not offer the 340B discounted prescription price to uninsured patients via these pharmacies…we...have no transparency into the behavior of these 340B contract pharmacies. None of the public companies reports any information about its participation in the 340B program.
- Health exchange disputes federal report (reviewjournal.com)
Nevada's health insurance exchange disputes a federal report claiming it misallocated funds for creating its program and recommending it consider refunding $893,000 to the Centers Medicare and Medicaid Services...A refund is not needed, the Silver State Health Insurance Exchange stated in a written response to the report from the Office of Inspector General, Department of Health and Human Services...We believe that this conclusion is based upon an erroneous interpretation of federal guidance regarding cost allocation accounting principles and the timing of cost allocation adjustments...It also pointed to comments from CMS that not only back up the state agency's take on the matter but are also included in the OIG's own report...In its report, however, the OIG was not swayed, stating it believes "our first recommendation is valid." The OIG cited the Nevada agency for using outdated data when it was establishing its marketplace despite the availability of updated data, having no internal controls, allowing insufficient staff oversight, and having no written policy on the allocation process...The Nevada marketplace should have used the updated, better data to update its cost allocation methodology...
- Can Hospitals Waive The Costs of Certain Drugs? (bna.com)OIG Policy Statement Regarding Hospitals That Discount or Waive Amounts Owed by Medicare Beneficiaries for Self-Administered Drugs Dispensed in Outpatient Settings (oig.hhs.gov)
Federal laws and regulations generally frown on hospitals providing Medicare patients with free or discounted medications, but a recent policy statement from the OIG appears to buck that trend. From now on, hospitals won’t face any administrative sanctions for waiving or discounting self-administered drugs (SADs) that are either administered or ingested in an outpatient setting and aren’t covered under Medicare Part B…policy statement was prompted by reports that some hospitals were charging Medicare patients for SADs, resulting in financial hardships for the patients. The hospitals believed that the anti-kickback statute required them to bill for the SADs, and at higher rates than patients would receive at retail pharmacies...Many hospitals are already waiving or discounting patient costs for SADs, as Medicare patients can end up paying more for the SADs than they would have if the drugs were dispensed at a retail pharmacy...