- First Gene-Transfer Therapy Approved for U.S. Market (ashp.org)Pioneering cancer drug, just approved, to cost $475,000 — and analysts say it’s a bargain (statnews.com)
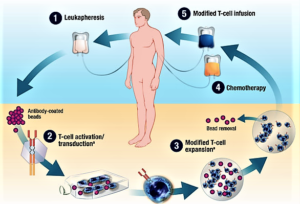
FDA...announced the approval of tisagenlecleucel, a first-in-class chimeric antigen receptor (CAR) T-cell immunotherapy consisting of genetically modified autologous T cells, for the treatment of B-cell precursor acute lymphoblastic leukemia (ALL) in children and young adults...After modification in the laboratory and infusion back into the patient, the CAR T cells target and eliminate both normal and malignant CD19-expressing B cells. The genetic modification enhances the initiation of T-cell activation and the persistence of the transformed T cells...Novartis will market tisagenlecleucel as Kymriah. Labeling for the product states that it is indicated in patients up to age 25 years with ALL that is refractory or in second or later relapse...Tisagenlecleucel has an FDA-required risk evaluation and mitigation strategy (REMS) program that includes elements to assure safe use. The labeling states that the immunotherapy is available "only through a restricted program."...
- The First CAR-T Drugs Have Left the Gate (fool.com)
Investors should keep an eye on this promising way to treat cancer...For all the talk about biotechs being nimble, it's a big pharma that looks like it'll be the first company to launch a chimeric antigen receptor T-cell (CAR-T) product...Novartis announced last week that the Food and Drug Administration accepted its application to market tisagenlecleucel-T...in patients with B-cell acute lymphoblastic leukemia who are relapsed and refractory to other therapies...A few days later, Kite Pharma completed its application for axicabtagene ciloleucel...Kite's application could be accepted early, putting it less than two months behind Novartis…Since CAR-T therapies are personalized treatments that have to be made individually for each patient, they're likely to be expensive to produce and therefore require a premium price. The first company to get a CAR-T therapy approved will set the price, which later companies may have to match unless they can justify a higher price with higher efficacy...With prices that will probably exceed those of current cancer treatments, investors should expect some pushback from insurers. One way Novarits and Kite can get around the cost issue is by offering money-back guarantees...Kite's and Novartis' CAR-T therapies are just the tip of the iceberg for this new way to treat cancer...